Ovarian adnexal mass present for which surgery is planned and not yet referred to an oncologist. The overall performance of ROMA and HE4 was better than that of CA125 but it was affected by pathologic types.
A Comparison Of Ca125 He4 Risk Ovarian Malignancy Algorithm Roma And Risk Malignancy Index Rmi For The Classification Of Ovarian Masses
This predicative probability algorithm is based on menopausal status and preoperative levels of HE4 and CA125 and is calculated as follows.
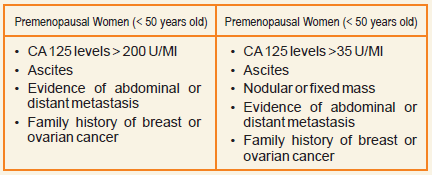
Roma blood test for ovarian cancer. ROMA Calculation is indicated for women who meet the following criteria. ROMA Risk of Ovarian Malignancy Algorithm - The risk of Ovarian Malignancy Algorithm ROMA test is intended to aid in assessing the risk of ovarian cancer in women with a pelvic mass based on the patients HE4 and CA125 levels and their menopausal status. Cancer antigen 125 CA125 has been shown to be elevated in most ovarian cancer cells but has a low specificity for ovarian malignancies156 Human epididymis protein 4 HE4.
OVA1 and OVERA are FDA-cleared blood tests for ovarian cancer with unparalleled abilities to detect ovarian cancer risk in women diagnosed with a pelvic mass. This test can be useful as a tumor marker to help guide treatment in women known to have ovarian cancer because a high level often goes down if. ROMA Risk of Ovarian Malignancy Algorithm is a predicative probability algorithm that classifies women with pelvic mass or ovarian cyst as being at high or low risk for epithelial ovarian cancer.
The CA-125 blood test measures the amount of a protein called CA-125 in the blood. ROMA is a qualitative serum test that derives a numerical score from the results of CA-125 the most widely accepted biomarker for ovarian cancer and HE4 blood tests plus menopausal status to identify patients presenting with an adnexal mass as being at high or low likelihood for having malignancy. It tests for CA125 transferring prealbumin apolipoprotein A1 and beta-2-microglobulin.
Ovarian cancer can recur in women who have been cancer-free for some time so this blood test is often part of a recall program where progress is monitored. The ROCA Test is intended for women who are between 50 and 85 years old and have been through menopause or between 35 and 85 years old with a family history of ovarian andor breast cancer are of Ashkenazi Jewish descent with a known family history of ovarian or breast cancer or have tested positive for BRCA1 BRCA2 or Lynch syndrome gene. For women with an adnexal mass OVA1 plus is a reflex process which first performs OVA1 and then performs OVERA if the OVA1 result is in the intermediate range.
Our study aims to give an update on the biological markers for diagnosing ovarian cancer specifically HE4 CA 125 RMI and ROMA algorithms. However a high false-negative rate was observed for early-stage cancers pre-menopausal patients and. Risk assessment for finding an ovarian malignancy during surgery in women who present with an adnexal mass The test is not intended as a screening or stand-alone diagnostic assay for.
The OVA1 test is a multiple biomarker blood test that was approved by the USFood and Drug Administration FDA in 2009. In Lennox et als independent study ROMA performed well for advanced ovarian cancer and high-grade serous histology by capturing 93 and 94 of these subtypes respectively. ROMA includes 2 recognized markers for ovarian cancer CA125 and HE4.
Women with ROMA levels above the cutoff have an increased risk of ovarian cancer. The Calculator is strictly to be used only in combination with test results from the Elecsys CA 125 II and HE4 immunoassays from. Ovarian cancer is the 5th leading cause of death for women with cancer worldwide.
Serum CA125 assay has low sensitivity in the early stages and can be increased in certain. Serum HE4 serum CA125 and ROMA index had better performance in the diagnosis of postmenopausal ovarian cancer than that of premenopausal ovarian cancer. The study is aimed at evaluating the performance of the predictive model ROMA Risk of Ovarian Malignancy Algorithm which utilizes the combination of human epididymis protein 4 HE4 and CA125 values to assess the risk of epithelial ovarian cancer EOC in women with a pelvic mass.
Many women with ovarian cancer have high levels of CA-125. In more than 70 of cases it is only diagnosed at an advanced stage. ROMA values must be interpreted in conjunction with an independent clinical and radiological assessment.
The individual biomarker test results are then transformed by computer software to generate an ovarian malignancy risk score. The Roche ROMA Risk of Ovarian Malignancy Algorithm calculator the Calculator is for the calculation of patients ROMA score using Elecsys CA 125 II and HE4 immunoassays from Roche. Serum HE4 serum CA125 and ROMA can be used to predict ovarian cancer.
The HE4 blood test is ordered to determine either the recurrence or progression of ovarian cancer. HE4 and ROMA have better.
